Prof’s Research Singled Out for Its Contribution to Understanding Ionic Liquids
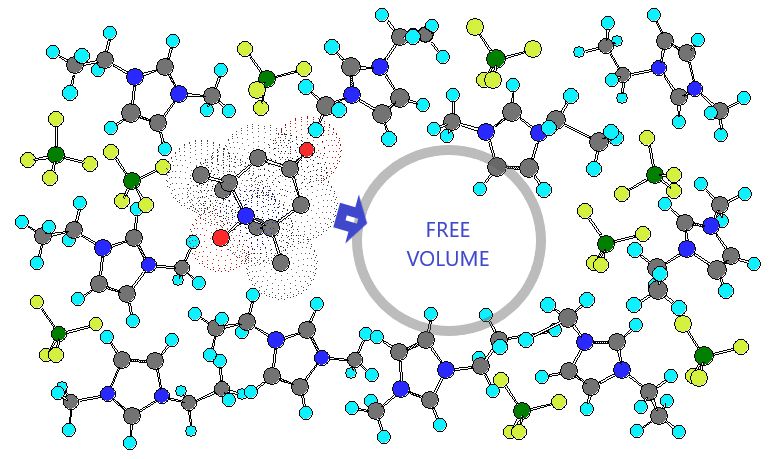
The illustration CSUN physicist Miroslav Peric used to demonstrate that the molecular dynamics, or chemical reactions, in ionic liquids can be manipulated by using the “free volume,” or the empty spaces, between the liquid molecules. Illustration courtesy of Miroslav Peric.
A paper by California State University, Northridge physicist Miroslav Peric has been singled out by the respected Journal of Chemical Physics for making a significant contribution to understanding ionic liquids — salts in a liquid state — which are increasingly being used as “green” substitutes for volatile organic solvents in such fields as solar cells, batteries, carbon capture, waste recycling and cellulose processing.
Peric and his colleague, physicist Dalibor Merunka with the Institut Ruder Boskovic in Croatia, were able to demonstrate that the molecular dynamics, or chemical reactions, in ionic liquids can be manipulated by using the “free volume,” or the empty spaces, between the liquid molecules. Their findings provide a foundation for future research and potential uses of ionic liquids in a variety of fields and industries.

Miroslav Peric
“Research of ionic liquids is only about 30 years old,” Peric said. “While in that time we’ve learned a lot and the applications for using ionic liquids have grown dramatically, there’s still a lot we don’t know. By learning more about how ionic liquids work, their potential applications grow.”
Peric said the recognition by The Journal of Chemical Physics — singling out the article about their research as an “Editor’s Pick” — was an “unexpected honor that says that what we’ve done has made a significant contribution to our field,” a contribution that the journal’s reviewers said is expected to be “significant, novel and of broad interest.”
The article, “An analysis of radical diffusion in ionic liquids in terms of free volume theory,” appeared in the Jan. 14, 2020, online issue of The Journal of Chemical Physics.
Peric, who has been studying ionic liquids for about 10 years, explained that ionic liquids are a new class of liquids that have only recently become a popular research topic due to their “interesting and unusual properties,” including low-vapor pressure, high-electric conductivity, high thermal stability and negligible flammability. Researchers are exploring ways to use them as substitutes for volatile organic solvents, chemicals that dissolve other chemicals. They are usually described as “designer,” non-volatile” and “modern.”
Table salt, which has the chemical name sodium chloride, is a solid at room temperature. But if it is heated up to 1,474 degrees Fahrenheit, table salt melts into a liquid. Sodium atoms are positively charged and are called cations, while chlorine atoms, called anions, are negatively charged.
“When table salt is liquid, it can be called an ionic liquid,” Peric said. “An ionic liquid is any liquid that is made of cations and anions.”
At room temperature, Peric said, there are no naturally occurring ionic liquids. The first room temperature ionic liquid was synthesized at the beginning of the 20th century. It was not until the 1970s and 1980s that chemists started to synthesize room-temperature ionic liquids, Peric said.
“Since then, the research on ionic liquids has been growing at a very fast pace,” he said.
“For example, while in 1998 there was no patent application involving their use, by 2015 ther were more than 800. During that same time, the number of published research articles has gone from a few tens to 5,000.”
Peric and Merunka used a newly developed spin probe electron paramagnetic resonance (EPR) Heisenberg spin exchange (HSE) technique — a method mostly developed by members of CSUN’s Electron Paramagnetic Resonance Laboratory — to study the bimolecular collisions between probe molecules — a group of atoms or molecules used to study the properties of other molecules or structures — in room temperature ionic liquids.
“The bimolecular collision of spin probe molecules is a good model for studying chemical reactions,” Peric said. “By studying these collisions, we can gain valuable insight into the molecular dynamics of chemical reactions in ionic liquids. A detailed understanding of this collision process and the role of the structure and dynamics of ionic liquid molecules may be useful in the design of ionic liquids, as well as their applications.”
In order to understand the bimolecular collisions of probe molecules in ionic liquids, Peric said, “we have to know how those molecules diffuse through the ionic liquid.”
To that end, Peric and Merunka used the Cohen-Turnbull free volume theory, which says that a fluid is not homogeneous at the molecular level because there is an empty space between liquid molecules, which is called the free volume.
Peric and Merunka applied the fact that the geometry of free volume is continuously changing due to thermal fluctuations. Once a void, or hole, larger than some critical size appears in the neighborhood of a probe molecule, the probe molecule jumps into the hole without any expense of energy. Then, the probe molecule remains in the hole until its next chance to move on.
The ability of the probe molecule to fill that free volume hole has implications for the potential manipulation and use of ionic liquids in such fields as solar cells, batteries, carbon capture, waste recycling and cellulose processing, Peric said.

 experience
experience